1. What is Thermodynamics??
Thermodynamics is the branch of science dealing with energy and various concepts and laws associated with it.
It also deals with laws concerned with the conversion of energy from one form to another.
It also deals the changes in the system caused due to the changes in variables.
2. What is system and surrounding??
The quantity of matter within a prescribed boundary under consideration is called the system, and everything external to the system is termed as the surroundings.
3. Closed system, Open system, and Adiabatic system???
With a closed system there is no interchange of matter between system and surroundings; with an open system there is such an interchange. No exchange of energy and matter in Adiabatic system.
4. State, Process , Cycle??
Certain variables like pressure , temp, volume etc are used to specify a system and is known as its state. Any changes in state that the system may undergo is known as a process. Any process or series of processes in which the system returns to its original condition or state is called a cycle.
5. Unite of Heat is Joule
6. The heat capacity??
There is a unit mass of substance. We need to raise the temp of it by 1 degree. The amount of heat reqd for that purpose is heat capacity.
"The heat capacity of a material is the amount of heat transferred to raise a unit mass of a material 1 deg in temperature."
7. specific heat ??
The ratio of the amount of heat transferred to raise unit mass of a material 1 deg to that required to raise unit mass of water 1 deg at some specified temperature is the specific heat of the material.
8. Latent heat ??
For pure substance, when some amount of heat is supplies at constant pressure, a change of state occurs without any changes in temperature. The amount of heat required for this is known as latent heat (of vaporisation/ sublimation /fusion)
9. First law of thermodynamics??
We have a system. When we add certain amount of heat internal energy of the system increases. When the system does some work it loses energy . So the net increase in internal energy of the system is equal to the amount of energy added t the system as heat minus the energy lost by the system by doing work.
"The increase in the internal energy of a system is equal to the amount of energy added by heating the system minus the amount lost as a result of the work done by the system on its surroundings."
U = Q - W or dU = dQ - dW
It is an expression of the principle of conservation of energy, states that energy can be transformed (changed from one form to another), but cannot be created or destroyed.
For reversible process dU = T dS - P dV
10. isobaric process??
process at constant pressure.
*An example would be to have a movable piston in a cylinder, so that the pressure inside the cylinder is always at atmospheric pressure, although it is isolated from the atmosphere.
11. isochoric process
process at constant volume.
- meaning that the work done by the system will be zero. It follows that, for the simple system of two dimensions, any heat energy transferred to the system externally will be absorbed as internal energy. An isochoric process is also known as an isometric process.
*An example would be to place a closed tin can containing only air into a fire.
12. isothermal process??
process at constant temperature.
* An example would be to have a system immersed in a large constant-temperature bath. Any work energy performed by the system will be lost to the bath, but its temperature will remain constant.
13. isentropic process??
process at a constant entropy.
- For a reversible process this is identical to an adiabatic process If a system has an entropy which has not yet reached its maximum equilibrium value, a process of cooling may be required to maintain that value of entropy.
14. adiabatic process??
it is a process in which there is no energy added or subtracted from the system by heating or cooling.
For a reversible process, this is identical to an isentropic process.
*We may say that the system is thermally insulated from its environment and that its boundary is a thermal insulator. If a system has an entropy which has not yet reached its maximum equilibrium value, the entropy will increase even though the system is thermally insulated.
15. When A system in thermodynamic equilibrium satisfies:
Extensive property is the property of a system which depend on the quantity of mateer eg : volume
As the quantity of matter increases or mass increases the volume also increases
Intensive property : Is the property of a system which do not depend on the mass of the system like temperature.
Specific property of a system is extensive property per unit mass. All of them are intensive property.
17.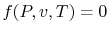 , which is known as an equation of state
, which is known as an equation of state
for ideal gas it is apprx as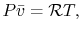
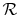 is the ``Universal Gas Constant,''
is the ``Universal Gas Constant,'' 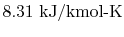
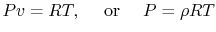 where R is
where R is 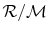
For air at room conditions,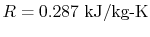
18. Zeroth Law
There are three bodies and, one and two are in equilibrium with each other and two and three are seperately in equilibrium with each other , then one and three are also in equilibrium with each other
So there exists a common property among these three bodies, that is the temperature and of course there exists a thermal equilibrium.
(to be contd....)
Thermodynamics is the branch of science dealing with energy and various concepts and laws associated with it.
It also deals with laws concerned with the conversion of energy from one form to another.
It also deals the changes in the system caused due to the changes in variables.
2. What is system and surrounding??
The quantity of matter within a prescribed boundary under consideration is called the system, and everything external to the system is termed as the surroundings.
3. Closed system, Open system, and Adiabatic system???
With a closed system there is no interchange of matter between system and surroundings; with an open system there is such an interchange. No exchange of energy and matter in Adiabatic system.
4. State, Process , Cycle??
Certain variables like pressure , temp, volume etc are used to specify a system and is known as its state. Any changes in state that the system may undergo is known as a process. Any process or series of processes in which the system returns to its original condition or state is called a cycle.
5. Unite of Heat is Joule
6. The heat capacity??
There is a unit mass of substance. We need to raise the temp of it by 1 degree. The amount of heat reqd for that purpose is heat capacity.
"The heat capacity of a material is the amount of heat transferred to raise a unit mass of a material 1 deg in temperature."
7. specific heat ??
The ratio of the amount of heat transferred to raise unit mass of a material 1 deg to that required to raise unit mass of water 1 deg at some specified temperature is the specific heat of the material.
8. Latent heat ??
For pure substance, when some amount of heat is supplies at constant pressure, a change of state occurs without any changes in temperature. The amount of heat required for this is known as latent heat (of vaporisation/ sublimation /fusion)
9. First law of thermodynamics??
We have a system. When we add certain amount of heat internal energy of the system increases. When the system does some work it loses energy . So the net increase in internal energy of the system is equal to the amount of energy added t the system as heat minus the energy lost by the system by doing work.
"The increase in the internal energy of a system is equal to the amount of energy added by heating the system minus the amount lost as a result of the work done by the system on its surroundings."
U = Q - W or dU = dQ - dW
It is an expression of the principle of conservation of energy, states that energy can be transformed (changed from one form to another), but cannot be created or destroyed.
For reversible process dU = T dS - P dV
10. isobaric process??
process at constant pressure.
*An example would be to have a movable piston in a cylinder, so that the pressure inside the cylinder is always at atmospheric pressure, although it is isolated from the atmosphere.
11. isochoric process
process at constant volume.
- meaning that the work done by the system will be zero. It follows that, for the simple system of two dimensions, any heat energy transferred to the system externally will be absorbed as internal energy. An isochoric process is also known as an isometric process.
*An example would be to place a closed tin can containing only air into a fire.
12. isothermal process??
process at constant temperature.
* An example would be to have a system immersed in a large constant-temperature bath. Any work energy performed by the system will be lost to the bath, but its temperature will remain constant.
13. isentropic process??
process at a constant entropy.
- For a reversible process this is identical to an adiabatic process If a system has an entropy which has not yet reached its maximum equilibrium value, a process of cooling may be required to maintain that value of entropy.
14. adiabatic process??
it is a process in which there is no energy added or subtracted from the system by heating or cooling.
For a reversible process, this is identical to an isentropic process.
*We may say that the system is thermally insulated from its environment and that its boundary is a thermal insulator. If a system has an entropy which has not yet reached its maximum equilibrium value, the entropy will increase even though the system is thermally insulated.
15. When A system in thermodynamic equilibrium satisfies:
- mechanical equilibrium (no unbalanced forces)
- thermal equilibrium (no temperature differences)
- chemical equilibrium.
Extensive property is the property of a system which depend on the quantity of mateer eg : volume
As the quantity of matter increases or mass increases the volume also increases
Intensive property : Is the property of a system which do not depend on the mass of the system like temperature.
Specific property of a system is extensive property per unit mass. All of them are intensive property.
17.
for ideal gas it is apprx as
For air at room conditions,
18. Zeroth Law
There are three bodies and, one and two are in equilibrium with each other and two and three are seperately in equilibrium with each other , then one and three are also in equilibrium with each other
So there exists a common property among these three bodies, that is the temperature and of course there exists a thermal equilibrium.
(to be contd....)
4
comments

